Companion Diagnostics
CDx solutions with GenDx: Your partner from start to finish
Introduction to CDx
Our Companion Diagnostics (CDx) services are designed to facilitate and support the pharmaceutical precision medicine revolution. New pharmaceutical therapies in the field of precision medicine require the presence of specific biomarkers or genetic profiles, which impact the eligibility or susceptibility of patients for the pharmaceutical therapy. For example, the genetic HLA profile of a patient can determine the response of a patient to a particular pharmaceutical therapy. The continuous progress in the field of precision medicine goes hand in hand with an increasing need for companion diagnostics that allow accurate patient selection.
GenDx, as a strong CDx partner, can support clinical study validation, ease navigating regulatory landscapes, and contribute to the success of a pharmaceutical therapy.
With companion diagnostics
Companion diagnostics are an important element of certain precision medicine therapies. A CDx can provide both healthcare provider and patient, with the specific biomarker information to assess whether particular therapies can benefit the patient, and in some cases, whether a therapies might be harmful to the patient.
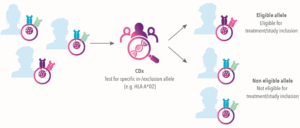
Companion diagnostics can also play a facilitating role in research, enabling faster, more tailored recruitment of eligible patients into clinical trials for precision therapies.
In short, companion diagnostics allow:
Targeted patient identification
Identify patients most likely to benefit from your therapy
Risk stratification
Identify individuals at increased risk for serious adverse reactions
Therapy monitoring
Monitor treatment response for optimized safety and efficacy

