Changes Under IVDR
GenDx has obtained the CE certificate under the new IVD Regulation (IVDR) for NGSgo® reagents and NGSengine® software (read more about this here).
Although the content of our kits remains the same under the IVDR, there are some important changes regarding:
√ Catalog numbers
√ Kit labels
√ Instructions for use
Changes in catalog numbers
NGSgo-AmpX v2 ● NGSgo-MX6-1 ● NGSengine
Because transitioning from IVDD to IVDR involves major administrative updates, catalog numbers have changed for several products*. Please take note of the following change in catalog numbers:
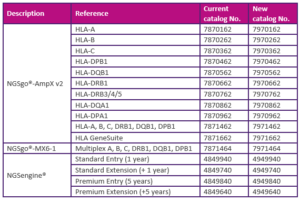
Although these catalog numbers have changed, the content of these products remains the same.
(*NGSgo-MX11-3 and NGSgo Library Full Kit are also CE marked under the IVDR. Catalog numbers for these products will remain the same, as these products were launched more recently and therefore complied with IVDR from the beginning).
When to start ordering the new catalog numbers?
NGSgo-AmpX v2 ● NGSgo-MX6-1 ● NGSengine
You can start ordering NGSgo-AmpX v2, NGSgo-MX6-1, and NGSengine under the new catalog numbers from June 15, 2022. To allow a transitioning period, ordering under the old catalog numbers remains possible until December 1, 2022. However, we would like to urge you to switch to the new numbers as soon as possible.
NGSgo-MX11-3 ● NGSgo Library Full Kit
We will start shipping NGSgo-MX11-3 and NGSgo Library Full Kit under the IVDR from June 15, 2022. As these catalog numbers do not change, this does not involve any ordering changes for you.
Changes in kit labels
New UDI code
Under the IVDR, product labels will contain a Unique Device Identifier (UDI). This is a barcode consisting of the following parts:
(01) product code (unique for each catalog number)
(10) lot number
(17) expiration date in YYMMDD format, where DD is 00 if day is not specified

You will find the UDI code on the kit labels for unambiguous identification of the kits in order to facilitate their traceability. For NGSengine, the UDI code will be displayed numerically on the splash screen of the software and will include the software version.
New label design
Labels have been slightly redesigned, including re-organization of symbols. New is a link to the location of the electronic version of the Instructions for Use: www.gendx.com/ifu.

Instructions for Use
New IFU versions
There are new versions of the Instructions for Use (IFU) for our IVDR products. The main changes in these versions include an updated intended purpose and additional product information. The laboratory protocols have not changed.
Next to English, these new versions of the IFUs will become available in various other European languages.
No printed IFU in kit
Under the IVDR, we are no longer including a printed version of the IFU in the kit. Instead, you can download the IFU from www.gendx.com/ifu.
If you do need an IFU in paper form, just send a request to support@gendx.com and we will ship a printed copy.
Need help or more information?
If you need any help or would like to have more information, please contact us at marketing@gendx.com


