During EFI 2023, in the GenDx Symposium, we presented a comparison study of chimerism monitoring by NGS and qPCR. In the article below, Maaike Rijkers PhD, Project Manager R&D at GenDx, shares the key highlights of this study.
In general practice, prior to hematopoietic stem cell transplantation, the recipient is first treated to remove most of the diseased cells. Then, hematopoietic stem cells originating from a donor are transferred to the recipient. After transplantation, the recipient is monitored over time in order to detect chimerism. An increasing number of recipient cells, also called increasing mixed chimerism (IMC), is important to detect as it may be a sign that the disease has returned (relapse). In addition to that, it could indicate graft rejection, minimal/measurable residual disease (MRD), or graft versus host disease (GVHD).
Chimerism can be measured using different methods. For all methods, pre-samples, obtained from both the recipient and the donor prior to transplantation, are used to determine informative markers. These informative markers are subsequently used to determine the chimerism percentage in samples obtained post transplantation (Figure 1).
Figure 1. Chimerism monitoring consists of two stages: 1) Genotyping, in order to determine informative markers. 2) In follow-up samples the informative markers are used to determine chimerism percentages over time.
The study was performed In collaboration with the Universitätsklinikum in Essen, we compared two different chimerism monitoring methods. Chimerism testing was performed in Essen using the qPCR-based method KMRtype, KMRtrack and KMRengine software.
Samples of 5 donor-recipient pairs, all including pre-samples and 5 to 7 follow up samples, were collected and sent to GenDx. At GenDx, the samples were tested using the NGS-based chimerism assay NGStrack using TRKengine software. In total, 10 pre- and 28 post-samples were blindly (without pre-knowledge on the qPCR results) tested. The results obtained with NGStrack were then reported, and subsequently compared to the KMR results.
With NGStrack, a high number of informative markers was obtained (between 17 and 25, of which 11 to 16 markers were recipient informative). Trends of all follow-up samples nicely overlap with the KMR data (see Figure 2), including samples with low (up to 0.1%) chimerism. A strong correlation of 0.9899 between KMR and NGStrack was observed (Figure 3).
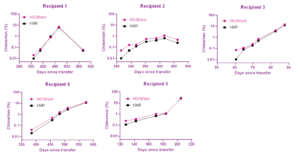
Figure 2. Chimerism percentages obtained with NGStrack (pink) and KMR (black).
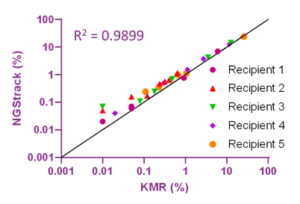
Figure 3. Correlation between results obtained with NGStrack and KMR. Different colours indicate different recipients.
The validated sensitivity of NGStrack is at least 0.5%. With this study, this sensitivity was confirmed. Additionally, we show that a sensitivity of 0.1% can be reached with NGStrack and identical trends are obtained when using NGStrack and KMR assays.
Maaike Rijkers PhD


